Understanding the Gibbs Free Energy Equation: A Comprehensive Guide
In our comprehensive guide, we delve into the intricacies of the Gibbs Free Energy Equation, a fundamental concept in thermodynamics that plays a pivotal role in understanding the feasibility of chemical reactions. The equation, named after the American scientist Josiah Willard Gibbs, is a mathematical representation of the maximum reversible work that a thermodynamic system can perform at constant temperature and pressure. It is a crucial tool in predicting the spontaneity of a process, a concept that is central to various scientific disciplines, including chemistry, physics, and biochemistry.
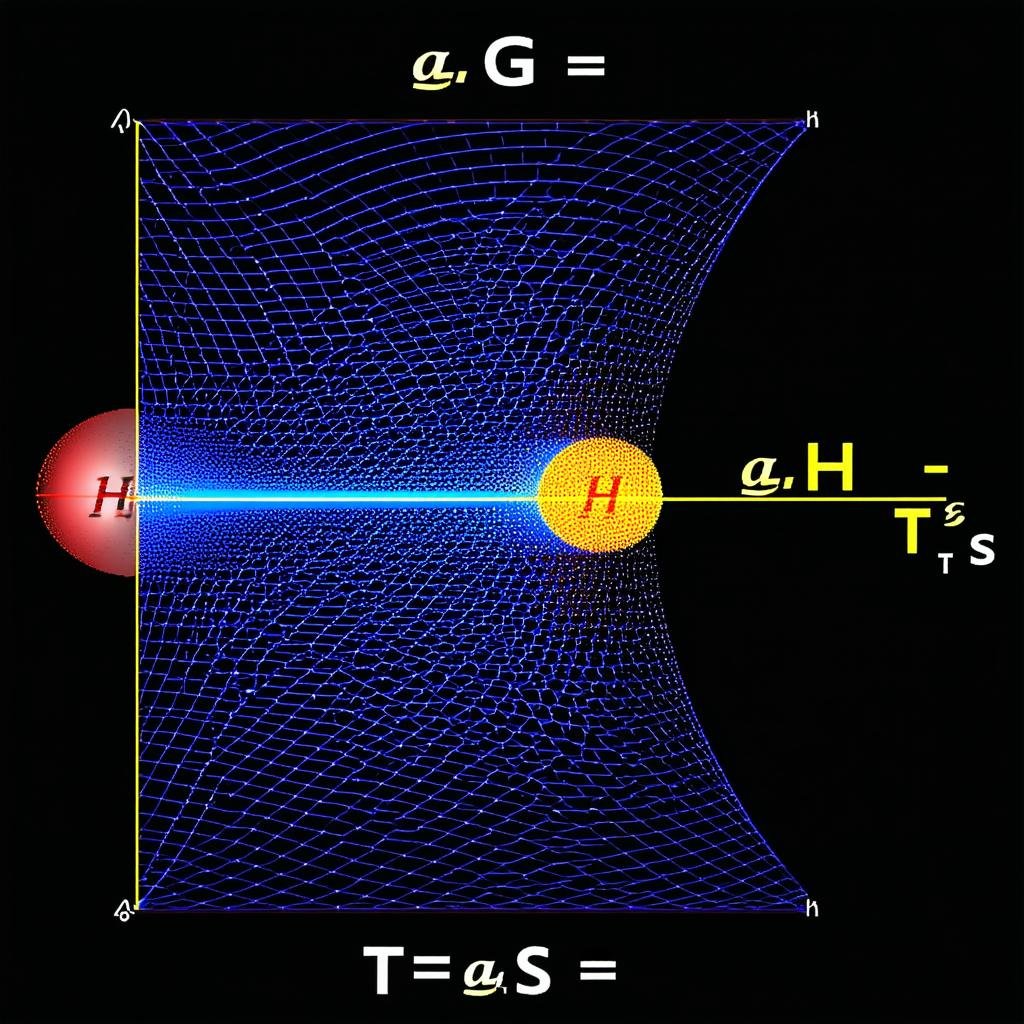
The Gibbs Free Energy (G) is defined as G = H – TS, where H represents the enthalpy, T is the absolute temperature, and S is the entropy of the system. Each of these components carries significant weight in the equation and contributes to the overall understanding of the system’s behavior. The enthalpy (H) is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create the system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.
The absolute temperature (T), measured in Kelvin, is a fundamental physical parameter that quantifies the thermodynamic system’s thermal energy. The entropy (S), on the other hand, is a measure of the system’s disorder or randomness. It is a state function, meaning its value depends solely on the current state of the system, not on how that state was achieved. The product of T and S in the Gibbs Free Energy Equation represents the energy unavailable for work in the system.
Understanding the Gibbs Free Energy Equation is not merely about comprehending the individual components but also about appreciating their interplay. The equation provides a quantitative method to determine whether a process will occur spontaneously. If the Gibbs Free Energy is negative, the process is spontaneous; if it’s positive, the process is non-spontaneous; and if it’s zero, the system is in equilibrium.
Moreover, the Gibbs Free Energy Equation is instrumental in understanding phase transitions, such as melting, freezing, and vaporization. It also plays a crucial role in the study of electrochemical cells, providing insights into the voltage that can be obtained from a galvanic cell and the minimum voltage required for an electrolytic cell to operate.
In conclusion, the Gibbs Free Energy Equation is a cornerstone of thermodynamics, offering profound insights into the spontaneity of processes and the balance between enthalpy, temperature, and entropy. By understanding this equation, we can predict and control the outcomes of chemical reactions, design efficient energy systems, and unravel the mysteries of natural phenomena.
Introduction to Gibbs Free Energy Equation
The Gibbs Free Energy Equation is a fundamental concept in thermodynamics, playing a pivotal role in predicting the feasibility of chemical reactions. It is named after Josiah Willard Gibbs, who introduced this concept in the late 19th century. The equation is a measure of the maximum reversible work that a thermodynamic system can perform at constant temperature and pressure.
The Mathematical Representation of Gibbs Free Energy
The Gibbs Free Energy (G) is mathematically represented as G = H – TS, where H is the enthalpy, T is the absolute temperature, and S is the entropy of the system. This equation is a balance between enthalpy, which represents the energy required for a reaction, and entropy, which is a measure of disorder or randomness in a system.
Understanding the Components of the Gibbs Free Energy Equation
Enthalpy (H)
Enthalpy is a thermodynamic quantity equivalent to the total heat content of a system. It is the sum of the internal energy and the product of pressure and volume. In chemical reactions, changes in enthalpy can either release or absorb energy, leading to exothermic or endothermic reactions, respectively.
Entropy (S)
Entropy is a measure of the randomness or disorder of a system. It is a fundamental concept in the second law of thermodynamics, which states that the total entropy of an isolated system can never decrease over time.
Temperature (T)
In the Gibbs Free Energy Equation, the temperature is always considered in Kelvin. It is a measure of the average kinetic energy of the particles in a system.
The Significance of Gibbs Free Energy
The Gibbs Free Energy Equation is crucial in determining whether a reaction is spontaneous or non-spontaneous. A negative Gibbs Free Energy indicates a spontaneous reaction, while a positive value suggests a non-spontaneous reaction.
Gibbs Free Energy and Equilibrium
At equilibrium, the Gibbs Free Energy is at its minimum. This state is characterized by a balance between the forward and reverse reactions, resulting in no net change in the concentrations of reactants and products.
Practical Applications of Gibbs Free Energy
The Gibbs Free Energy Equation is widely used in various fields, including chemistry, physics, engineering, and biology. It helps in predicting the behavior of chemical reactions, designing energy-efficient systems, and understanding biological processes.
Conclusion
The Gibbs Free Energy Equation is a cornerstone of thermodynamics, providing valuable insights into the spontaneity and equilibrium of chemical reactions. Understanding this equation is crucial for anyone studying or working in fields related to thermodynamics. It is a testament to the genius of Josiah Willard Gibbs and his enduring contribution to science.
FAQs
Q1: What does a negative Gibbs Free Energy indicate?
A negative Gibbs Free Energy indicates a spontaneous reaction.
Q2: What is the relationship between Gibbs Free Energy and equilibrium?
At equilibrium, the Gibbs Free Energy is at its minimum.
Q3: How is the Gibbs Free Energy Equation used in practical applications?
It is used in predicting the behavior of chemical reactions, designing energy-efficient systems, and understanding biological processes.
Q4: What does the ‘T’ in the Gibbs Free Energy Equation represent?
‘T’ represents the absolute temperature, measured in Kelvin.
Q5: Who introduced the concept of Gibbs Free Energy?
The concept of Gibbs Free Energy was introduced by Josiah Willard Gibbs.